Iso 13485 Sop Documents
Posted : adminOn 3/30/2018
ISO 13485 is an effective solution to meet the global requirements for a quality management system (QMS). The adoption of the ISO 13485 standard provides a convenient base for manufacturers to deal with rules and responsibilities and demonstrate a commitment to safety and quality of medical devices. ISO 13485 is a stand-alone QMS standard, derived from the ISO 9000 quality management standard internationally recognized and accepted standard. ISO 13485 adapts the model based on ISO 9000 process for a regulated environment for medical device manufacturing. Key Importance of ISO 13485: are important for designers, medical device manufacturers and distributors. The Getaway Plan Other Voices Other Rooms Zip here.
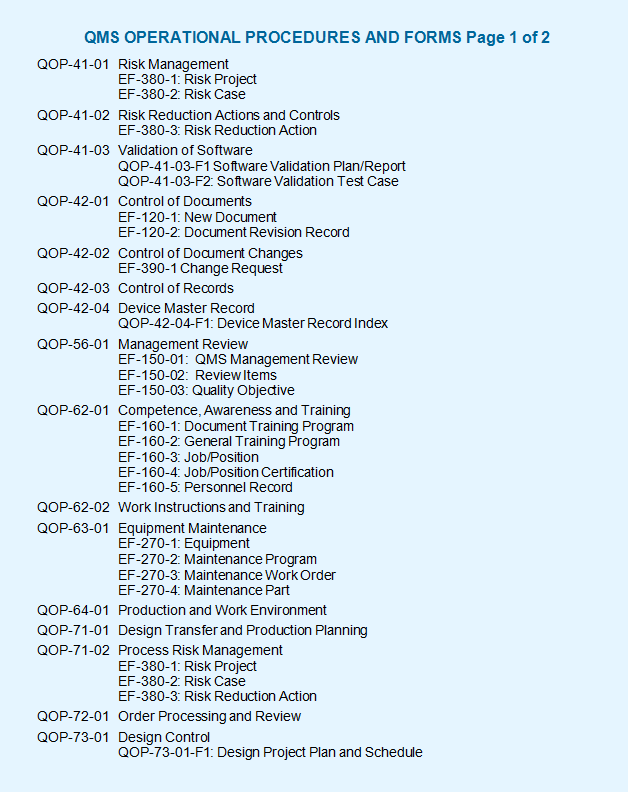
ISO 13485 Store >ISO Requirements >ISO 13485 Documentation Requirements. Six Procedures. Control of Documents (4. Program Of Dragon Ball Z Mugen Edition. 2.3) Control of Records (4.2.4).
In addition, suppliers and service providers can improve the marketing of an organization increasingly manufacturers require certification to do business with a supplier. When it comes to the manufacture of medical devices, patient safety depends greatly on the quality and consistency of medical products, and ensure the efficiency, control and maintenance of your quality management system is essential for customers, stakeholders, patients and users, and regulators. The value of ISO 13485 is not only in implementation but also in providing a tool for a thorough audit to test the effectiveness of the system.
It provides the manufacturer with a higher level of confidence in the ability to consistently achieve and maintain compliance with regulatory requirements. In addition, it can help minimize surprises and failures that could affect patient safety and harm the reputation of a manufacturer. The beneficial outputs of an effective ISO 13485 are as follows: • Meaningful feedback on the effectiveness of the quality management system. • Confidence in regulatory compliance. • Identification of areas requiring attention. • Confirmation that the best practice is achieved.